
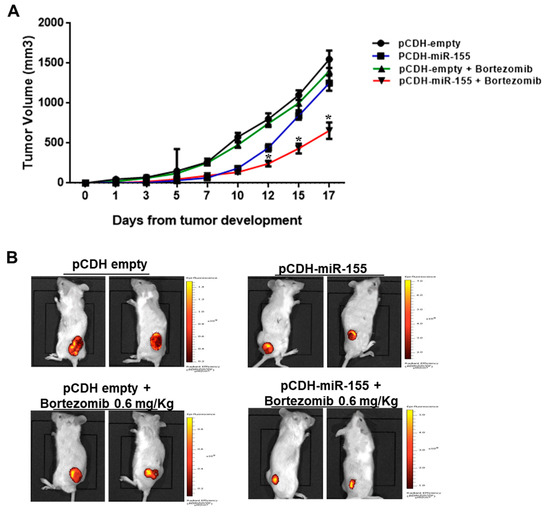
This model may be used to study human transplantation immunobiology in vivo. In conclusion, we show that intraperitoneal injection of 30–40 × 10 6 human splenocytes into SCID/beige mice induces a quick and functional engraftment of human T, B and NK cells with no risk of GVHD. When more than 40 × 10 6 cells were injected, GVHD occurred with increasing frequency. When less than 30 × 10 6 cells were injected, the reconstitution was variable. The reconstitution was functional because graft rejection was observed after transplantation of human allogeneic tissues. In lymphoid tissues, the same lymphocytic subpopulations were detected and in addition some antigen presenting cells. Human T, B and NK cells as well as human IgG were present in peripheral blood. A reproducible reconstitution was obtained with intraperitoneal injection of 30–40 × 10 6 spleen cells. The mice were sacrificed 4 weeks after the injection and the engraftment in lymphoid organs was studied. Reconstitution of a human immune system was monitored weekly by the presence of human cells and IgG in peripheral blood. Single intraperitoneal injections of 5–100 × 10 6 splenocytes were performed into SCID/beige mice. After institutional authorisations and informed consent of relatives, a piece of spleen was obtained from cadaveric organ donors and the splenocytes were isolated and cryopreserved for later use. SCID/beige mice are at the same time deficient for adaptive and innate immunity and the objective of this study was to develop a safe and efficient way to achieve human lymphocyte engraftment in these mice using human spleen cells. Reconstitution with human cells can be achieved using large numbers of peripheral blood lymphocytes, but levels of engraftment are poor and graft versus host disease (GVHD) frequently occurs. In this chapter, we discuss the current state of development of these strains of mice, the remaining deficiencies, and how approaches used to increase the engraftment and function of human hematolymphoid cells in CB 17-scid mice and in previous models based on NOD-scid mice may enhance human hematolymphoid engraftment and function in NOD-scid IL2rgamma(null) mice.Models of severe combined immuno-deficient (SCID) mice reconstituted with a competent human immune system represent a valuable tool for the study of human immune responses in vivo. Similarly, localized iMNP depletion by clodronate-encapsulated liposomes into C57BL/6, BALB/c, and CB.17/SCID mice also increased DSS colitis severity, as indicated by increased histopathology, weight loss, rectal bleeding, decreased stool consistency, and colon length compared with M/DC-intact, DSS-treated mice. These new strains of immunodeficient IL2rgamma(null) mice are now being used for studies in human hematopoiesis, innate and adaptive immunity, autoimmunity, infectious diseases, cancer biology, and regenerative medicine. Third, immunodeficient mice bearing a targeted mutation in the IL-2 receptor common gamma chain (IL2rgamma(null)) were developed independently by four groups between 20, and a major increase in the engraftment and function of human hematolymphoid cells as compared with NOD-scid mice has been reported. NOD-scid mice have been the "gold standard" for studies of human hematolymphoid engraftment in small animal models over the last 10 years.
Second, NOD-scid mice were developed and their enhanced ability to engraft with human hematolymphoid tissues as compared with CB17-scid mice was reported in 1995.
First, CB 17-Prkdc(scid) (abbreviated CB 17-scid) mice were discovered in 1983, and engraftment of these mice with human fetal tissues (SCID-Hu model) and peripheral blood mononuclear cells (Hu-PBL-SCID model) was reported in 1988. Female CB.17-SCID Mice Mouse Strain: CB17/ Icr-Prkdcscid/IcrIcoCrl Data presented as median, n7 or 8/group. Progress in development of small animal models for the in vivo investigation of human hematopoiesis and immunity has seen three major breakthroughs over the last three decades. There is a growing need for effective animal models to carry out experimental studies on human hematopoietic and immune systems without putting individuals at risk.


 0 kommentar(er)
0 kommentar(er)
